High levels of calcium in your blood-- nausea vomiting constipation increased thirst or urination muscle weakness bone pain confusion lack of energy or feeling tired. 471-34-1 is produced industrially by the decomposition of limestone to calcium oxide followed by subsequent recarbonization or as a by-product of the Solvay process which is used to make sodium carbonate.
Limestone Calcium Carbonate Caco3 Uses Preparation Properties Formula Structure Of Calcium Carbonate
Its most common natural forms are chalk limestone and marble produced by the sedimentation of the shells of small fossilized snails shellfish and coral over millions of years.

. This is not a complete list of side effects and others may occur. Which of the following is true of the two body forms found in cnidarians polyp and medusa. Limestone is formed _____ by organisms such as coral that extract calcium cabonate from the water to form their shells and skeletons.
The correct answer is Limestone. Ca is calcium C is carbon and O is oxygen. The value of x in FeNH42SO42.
Here are 15 foods that are rich in calcium many of which are nondairy. H2O can be found by determining the amount in mol of sulfate in the compoundA 0982 g sample was dissolved in wate. Calcium carbonate is a chemical inorganic compound having the chemical formula CaCO3It is also one of the most popular chemicals which is encountered first in school classrooms where the use of chalk which is a form of CaCO3 is found.
Calcium carbonate is a chemical compound with the formula Ca CO 3It is a common substance found in rocks as the minerals calcite and aragonite most notably as limestone which is a type of sedimentary rock consisting mainly of calcite and is the main component of eggshells gastropod shells shellfish skeletons and pearlsCalcium carbonate is the active ingredient in. Treatment of heartburn andor GERD symptomsThe client experiences persistent heart that. Most of it is mined from quarries and pure sources of calcium carbonate can be extracted and used for foods and pharmaceuticals.
Calcium carbonate is a major constituent of pancreatic stones consisting of ca. Yes calcium carbonate has three elements. Calcium carbonate is CaCO3.
Cave formations like stalagmites. A soft very fine-grained limestone formed via the accumulation of the calcium carbonate remains of microorganisms is known as _____. Does calcium carbonate contain of three elements.
In this mixture 089 of the mass is contributed by the NaCl. Precipitated calcium carbonate CAS. Low carbon dioxide and warmer temperatures b.
Limestone is a form of calcium carbonate. Lots of carbon dioxide and colder temperatures c. Eggshells and seashells are calcium carbonate.
It is available in various forms such as limestone marble and more. You find it in chalk limestone and marble its also present in the shells of shellfish explains the Industrial Minerals Association of America. Calcium carbonate or CaCO3 comprises more than 4 of the earths crust and is found throughout the world.
Calcite chalk eggshells limestone marble and Tums. R and excess BaCl2 aq was added. Which of the following shows the chemical formula for calcium carbonate.
Calcium carbonate can be found in rocks snails pearls and more. The normal function of pancreatic secretion is to contribute to the. It is found in the crust of the earth.
Calcium carbonate is most likely to dissolve in water with which characteristics. What mass of NaCl is found in 450. DXA scan reveals 35 bone mass loss calcium carbonate is used as a mineral replacement supplement to prevent further bone loss and reduce risk of fractures.
It is also used as fillers in cosmetics. Precipitated calcium carbonate is purer than ground calcium carbonate and has different and tailorable handling properties. Dead calcium carbonate skeletons accumulate below it.
It is a white insoluble powder-like substance which occurs naturally in minerals chalk marble limestone calcite shells pearl etc. Low pressure and warmer temperatures e. 95 calcite and is found occasionally in salivary stones and many pigment gallstones since these three gastrointestinal secretions have high pH values and contain high hydrogen carbonate concentrations.
The precipitate of BaSO4 was separated and dried and found to weight 117g. Reason Calcium Carbonate Diagnosed with osteoporosis. Low pressure and colder temperatures.
Common side effects may include. Calcium carbonate CaCO 3 Limestone and calcite are calcium carbonate. Seeds are tiny nutritional powerhouses and many are high in calcium including poppy sesame celery and chia.
Of calcium is 12 g. Choose the response that includes all the items listed below that are pure substances. Besides the so-called ground calcium carbonate GCC which is milled.
Although all three forms are identical in chemical terms they differ in many other. It is a chemical compound with the chemical formula CaCO3. Which of the following features is composed of calcium carbonate.
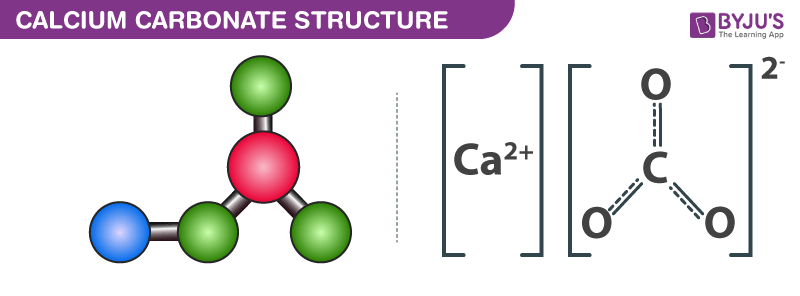
Medicinally it is used as an antacid or as a calcium supplement. Calcium carbonate contains 120 calcium by mass. A calcium carbonate molecule has 1 calcium atom 3 oxygen atoms and 1 carbon atom.
Because of this there are six common names for the element. Mineral and electrolyte replacement supplements. A Calculate the amount in mol of BaSO4 in the 117g of precipitate.
Calcium carbonate is found throughout the world as part of the earths crust. Other Different forms of calcium carbonate are Chalk and Marble. Larger food items are first broken down with digestive enzymes before the particles are engulfed by cells.
Calcium carbonate CaCO 3 is a substance widely used for various purposes for example as a filler and pigment material not only in paper plastics rubbers paints and inks but also in pharmaceutics cosmetics construction materials and asphalts and as a nutritional supplement in animal foods 1. How many grams of calcium carbonate are needed to provide the RDA of calcium. Calcium carbonate is a chemical compound with the formula CaCO3.
Calcium chloride CaCl 2 Calcium chloride can be found. Eggshells and seashells are calcium carbonate. It is a common substance found in rocks as the minerals calcite and aragonite most notably as limestone which is a type of sedimentary rock.
They share the same basic morphology - mouth gastrovascular cavity and tentacles. Lots of carbon dioxide and warmer temperatures d.

Limestone Calcium Carbonate Caco3 Uses Preparation Properties Formula Structure Of Calcium Carbonate

Calcium Carbonate Definition Formula Preparation Structure Properties

0 Comments